Vitamin C Ascorbic Acid CAS 50-81-7 Medicine Vitamins Food And Feed Additive Nutritional
Vitamin C Ascorbic Acid |
CAS No.: 50-81-7 |
Purity: 99% |
Molecular weight: 176.12 g/mol |
Molecular formula: C6H8O6 |
Appearance:White to light yellow crystals or powder |
Package: 1g,10g,100g,1000g |

Vitamin C Ascorbic Acid CAS 50-81-7 Medicine Vitamins Food And Feed Additive Nutritional
MF:C6H8O6
Purity:99%
Package:25kg/drum
Color:White
Laisser un message
Nous vous rappellerons bientôt!
Chère,
Je suis intéressé par Vitamin C Ascorbic Acid CAS 50-81-7 Medicine Vitamins Food And Feed Additive Nutritional, pouviez-vous m'envoyer plus de détails tels que le type, la taille, le MOQ, le matériel, etc.
Merci !
En attendant votre réponse.
50-81-7 Food Additives
,Food Additives 99%
,Food grade Ascorbic Acid
Vitamin C Ascorbic Acid CAS 50-81-7 Medicine Vitamins Food and feed additive Nutritional
Description:
Ascorbic acid, a water-soluble dietary supplement, is consumed by humans more than any other supplement. The name ascorbic means antiscurvy and denotes the ability of ascorbic to combat this disease. Vitamin C is the l-enantiomer of ascorbic acid. Ascorbic acid deficiency in humans results in the body’s inability to synthesize collagen, which is the most abundant protein in vertebrates.
L-Ascorbic acid is a naturally occurring electron donor and therefore serves as a reducing agent. It is synthesized from glucose in the liver of most mammalian species, excluding humans, non-human primates, or guinea pigs who must obtain it through dietary consumption. In humans, L-Ascorbic acid acts as an electron donor for eight different enzymes, including those related to collagen hydroxylation, carnitine synthesis (which aids in the generation of adenosine triphosphate), norepinephrine synthesis, tyrosine metabolism, and amidating peptides. L-Ascorbic acid demonstrates antioxidant activity that may be of some benefit for reducing the risk of developing chronic diseases such as cancer, cardiovascular disease, and cataracts.
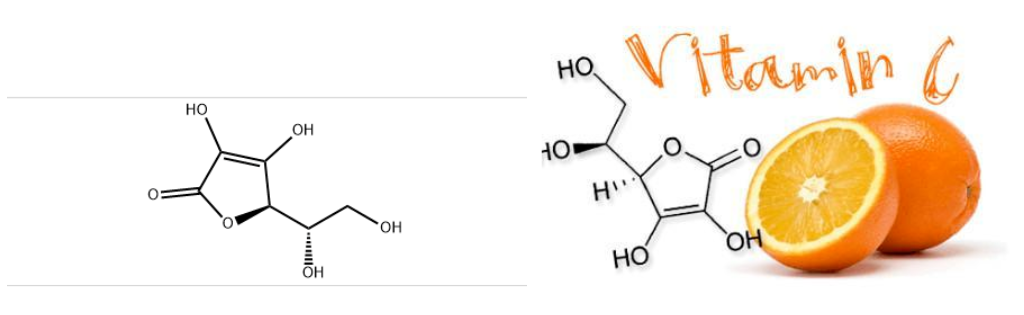
Basic Parameters:
| Synonym | Antiscorbutic vitamin |
| Melting point | 190-194 °C (dec.) |
| Storage temp | Store at +5°C to +30°C. |
| Solubility | H2O: 50 mg/mL at 20 °C, clear, nearly colorless |
| Stability | Stable. May be weakly light or air sensitive. Incompatible with oxidizing agents, alkalies, iron, copper. |

History:
Vitamin C is a general term for compounds having ascorbic acid activity, including ascorbic acid, dehydroascorbic acid, and its isomers.
The understanding of vitamin C has gone through a long and painful process. Although the relationship between scurvy and stored food is obvious, but the treatments of this disease have been misguided. By 1601, British armed Captain James Lancaster discovered the disease on the ship of the East India Company and regarded the scurvy as “rot,” which could be made tissue alkaline.
At the early stage of the nineteenth century, the understanding and treatment of scurvy had developed to a right approach. The exposition of scurvy etiology and metabolic theory took more than a century.
By the early stage of the twentieth century, inspired by the animal model of beriberi, researchers in the Christchurch Oslo University discovered one animal that could suffer scurvy accidentally and then established a valuable scurvy animal model. This experiment demonstrated that the extract isolated from lemon had antiscurvy activity. Until 1932, many research groups obtained the anti-scurvy crystal from different plants and identified the crystal as ascorbic acid vitamin C. Next year, the chemical structure of ascorbic acid was elucidated, and then its artificial synthesis was accomplished.

Picture 2
Uses:Vitamin C is a well-known anti-oxidant. Its effect on free-radical formation when topically applied to the skin by means of a cream has not been clearly established. The effectiveness of topical applications has been questioned due to vitamin C’s instability (it reacts with water and degrades). Some forms are said to have better stability in water systems. Synthetic analogues such as magnesium ascorbyl phosphate are among those considered more effective, as they tend to be more stable. When evaluating its ability to fight free-radical damage in light of its synergistic effect with vitamin e, vitamin C shines. As vitamin e reacts with a free radical, it, in turn, is damaged by the free radical it is fighting.
>Vitamin C comes in to repair the free-radical damage in vitamin e, allowing e to continue with its free-radical scavenging duties. Past research has indicated that high concentrations of topically applied vitamin C are photoprotective, and apparently the vitamin preparation used in these studies resisted soap and water, washing, or rubbing for three days. More current research has indicated that vitamin C does add protection against uVB damage when combined with uVB sunscreen chemicals. This would lead one to conclude that in combination with conventional sunscreen agents, vitamin C may allow for longer-lasting, broader sun protection.
Again, the synergy between vitamins C and e can yield even better results, as apparently a combination of both provides very good protection from uVB damage. However, vitamin C appears to be significantly better than e at protecting against uVA damage. A further conclusion is that the combination of vitamins C, e, and sunscreen offers greater protection than the sum of the protection offered by any of the three ingredients acting alone.
Vitamin C also acts as a collagen biosynthesis regulator. It is known to control intercellular colloidal substances such as collagen, and when formulated into the proper vehicles, can have a skin-lightening effect. Vitamin C is said to be able to help the body fortify against infectious conditions by strengthening the immune system. There is some evidence (although debated) that vitamin C can pass through the layers of the skin and promote healing in tissue damaged by burns or injury. It is found, therefore, in burn ointments and creams used for abrasions.
Vitamin C is also popular in anti-aging products. Current studies indicate possible anti-inflammatory properties as well.
Reference
Chemical Book www.chemicalbook.com
Produits recommandés










