Ornithine-Crosslinked Sodium Hyaluronate Powder
Ornithine-Crosslinked Sodium Hyaluronate Powder |
Purity: Cosmetic-grade ingredients typically have a purity of ≥90%, while pharmaceutical-grade may reach up to ≥98%. |
Molecular weight: The natural molecular weight range is quite broad (e.g., low molecular weight: ~10 kDa; high molecular weight: ~1-5 MDa). |
Appearance: Typically white or pale yellow powder, with particle sizes varying depending on the manufacturing process (such as micrometer or nanometer scale) |
Package: 1g,10g,100g,1000g |

Ornithine-Crosslinked Sodium Hyaluronate Powder
Description
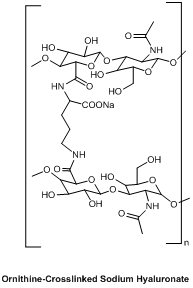
Ornithine-Crosslinked Sodium Hyaluronate is a chemically modified hyaluronic acid (HA) derivative engineered through covalent crosslinking with ornithine, a non-proteinogenic amino acid. This modification enhances the polymer’s viscoelasticity, biodegradation resistance, and longevity in vivo compared to linear HA. The product is supplied as a sterile, lyophilized powder for reconstitution with appropriate solvents.
Laisser un message
Nous vous rappellerons bientôt!
Chère,
Je suis intéressé par Ornithine-Crosslinked Sodium Hyaluronate Powder, pouviez-vous m'envoyer plus de détails tels que le type, la taille, le MOQ, le matériel, etc.
Merci !
En attendant votre réponse.
Product Name: Ornithine-Crosslinked Sodium Hyaluronate Powder
INCI Name: Sodium Hyaluronate Crosspolymer
Description
Ornithine-Crosslinked Sodium Hyaluronate is a chemically modified hyaluronic acid (HA) derivative engineered through covalent crosslinking with ornithine, a non-proteinogenic amino acid. This modification enhances the polymer’s viscoelasticity, biodegradation resistance, and longevity in vivo compared to linear HA. The product is supplied as a sterile, lyophilized powder for reconstitution with appropriate solvents.
Key Features
High Elasticity & Cohesivity: Optimized for tissue support and volumization.
Biocompatible & Bioresorbable: Degrades naturally via hydrolysis and enzymatic action.
Customizable Viscosity: Rheological properties adjustable via crosslinking density.
Sterile & Pyrogen-Free: Meets ISO 13485 and USP standards for medical devices.
Intended Use
Dermal Fillers: For mid-to-deep dermal implantation to correct moderate-to-severe wrinkles, folds, and volume loss (e.g., nasolabial folds, cheeks).
Joint Lubrication: Intra-articular injection for osteoarthritis management (pending regulatory approval).
Wound Healing: Topical or injectable formulations for tissue regeneration.
Composition
| Component | Specification |
|--------------------------|---------------------------------------- |
| Sodium Hyaluronate | Crosslinked with ornithine [XX%] |
| Residual Ornithine | ≤ [X] ppm (per batch analysis) |
| Moisture Content | ≤ 2.0% (Karl Fischer method) |
| Endotoxin Level | < 0.05 EU/mg (LAL test) |
Reconstitution Guidelines
1. Solvent: Use sterile phosphate-buffered saline (PBS) or lidocaine HCl (0.3–1.0%) for clinical applications.
2. Ratio: 1:1 to 1:2 (powder:solvent) depending on desired viscosity (e.g., 20 mg/mL for high cohesivity).
3. Mixing: Gently swirl (avoid vortexing) at room temperature for 5–10 minutes until fully dissolved.
Storage & Handling
- Storage: 2–8°C in original sealed vial. Protect from light and moisture.
- Shelf Life: 24 months unopened. Use immediately after reconstitution (stable for ≤24 hours at 4°C).
- DO NOT FREEZE reconstituted product.
Safety & Precautions
- Contraindications: Hypersensitivity to hyaluronic acid, gram-positive bacterial protein allergy, or active infection at injection site.
Adverse Reactions: Temporary erythema, swelling, or tenderness (resolve within 7–14 days).
Post-Treatment Care: Avoid UV exposure, excessive heat, or strenuous activity for 48 hours.
Regulatory Status
- CE Marked under Class III Medical Device (EU).
- For investigational use in the USA (not FDA-approved).
Packaging
- Form: Lyophilized powder in sterile glass vials.
- Sizes: 10 mg, 20 mg, 50 mg per vial.
This product is intended for use by licensed healthcare professionals. Always follow local regulations and clinical guidelines.*
Let me know if you need adjustments for specific regulatory requirements or application details!
Produits recommandés










